QIAamp Virus BioRobot 9604 Kit
在BioRobot 9604工作站上,从无细胞体液中自动纯化病毒DNA和RNA
在BioRobot 9604工作站上,从无细胞体液中自动纯化病毒DNA和RNA

目录编号 / ID. 965662
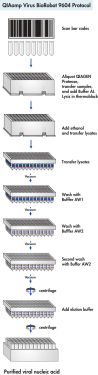
QIAamp Virus BioRobot 9604 Kit使用经验证的QIAamp技术在BioRobot 9604工作站上自动纯化病毒核酸。全程自动化运行无需手动操作,2.5小时内即可处理96个样本,其间完成条形码读取和过程存档。
QIAamp 96孔板可确保RNA和DNA回收的孔间一致性,能可靠用于下游应用。使用QIAamp技术从无细胞体液中制备的病毒RNA和DNA可即时用于PCR和印迹实验。
该流程无需苯酚-氯仿抽提。核酸特异性结合到QIAamp硅胶膜上,污染物流出。通过高效洗涤完全去除二价阳离子和蛋白质等PCR抑制剂,再用水或试剂盒中的缓冲液洗脱结合在离心柱上的核酸。
试剂盒提供优化的缓冲液高效裂解样本,稳定核酸并促进其选择性结合到QIAamp硅胶膜上。裂解液中添加乙醇并上样到QIAamp 96孔板。在BioRobot 9604工作站上使用洗涤缓冲液去除杂质,最后用低盐缓冲液洗脱得到即时可用的核酸(参见" Protocol")。
该试剂盒2.5个小时内即可从96个无细胞体液样本中纯化得到高纯度的RNA和DNA,适用于从人和动物病毒中分离核酸。QIAamp样本制备技术完全得到验证认可。
| 特点 | 规格 |
|---|---|
| applications | PCR, blotting |
| forautomatedprocessing | BioRobot 9604 Workstation |
| mainsampletype | Serum, plasma |
| elutionvolume | 50 µl |
| format | 96-well plates |
| processing | Automated |
| purificationoftotalrnamirnapolyamrnadnaorprotein | Viral DNA, viral RNA |
| technology | Silica technology |
| sampleamount | <200 µl |
| timeperrunorperprep | <2.5 hours |
| yield | >90% recovery |