In vitro diagnostic를 목적으로 사용하기 위해 human plasma와 serum에서 viral nucleic acid를 분리정제할 수 있습니다
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
QIAamp DSP Virus Kit
카탈로그 번호 / ID. 60704
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
특징
- Universal nucleic acid purification system compatible with other IVD products
- Qualitative와 quantitative NAT assays에서 핵산의 고감도 detection
- 500 ul 샘플로 부터 20 또는 60 ul의 nucleic acid로 농축 가능함
- 신속한 분리 정제, cross-contamination 위험의 최소화
제품 세부 정보
The QIAamp DSP Virus Kit uses well-established and convenient QIAamp technology for simultaneous purification of viral DNA and RNA for in vitro diagnostic use.
성능
Viral nucleic acids purified using the QIAamp DSP Virus Kit are ready to use in sensitive downstream applications, such as those based on enzymatic amplification or other modification, including PCR and RT-PCR.
원리
The QIAamp DSP Virus Kit uses well-established and convenient QIAamp technology for simultaneous purification of viral DNA and RNA. The QIAamp silica-based membrane binds nucleic acids in the lysed sample, while the rest of the lysate is rapidly removed by vacuum pressure. The bound nucleic acids are efficiently washed to remove contaminants and then eluted in a volume of 20 µl or 60 µl.
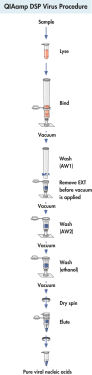
절차
그림 참조
응용 분야
Viral nucleic acids can be purified from plasma or serum samples. Samples can contain the anticoagulants citrate or EDTA, and can be either fresh, lyophilized, or frozen (provided they were not thawed and refrozen).
The QIAamp DSP Virus Kit provides purification of nucleic acids from a wide range of viruses. The kit is compatible with a wide range of upstream sample collection systems and downstream applications, and therefore can be easily integrated into diagnostic workflows.
지원되는 데이터 및 수치
QIAamp DSP Virus Kit procedure.
사양
| 특징 | 사양 |
|---|---|
| applications | Downstream detection procedures in molecular diagnostics, such as PCR |
| elutionvolume | 20 µl, 60 µl |
| mainsampletype | Serum, plasma |
| cefdaivdcompatible | CE/IVD |
| format | Sample tubes |
| processing | Manual (vacuum) |
| purificationoftotalrnamirnapolyamrnadnaorprotein | Viral DNA, viral RNA |
| sampleamount | 500 µl |
| technology | Silica technology |
| yield | Varies |