✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QIAcuity Probe PCR Kit (5 ml)
Cat no. / ID. 250102
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- For singleplex and up to 5-plex reactions
- 4x concentrated Master Mix for loading more sample
- Optimized for microfluidic use in QIAcuity Nanoplates
- Less unspecific PCR amplification
- REACH compliant
Product Details
The QIAcuity Probe PCR Kit contains a 4x concentrated, ready to use Master Mix optimized for microfluidic use in the QIAcuity Nanoplates. The kit enhances the specificity and efficiency of probe-based digital PCR to provide accurate, singleplex, or up to 5-plex analysis. The QIAcuity Probe Master Mix is optimized to increase specificity for accurate quantification of gDNA or cDNA on the QIAcuity dPCR instruments.
The kit works in conjunction with the QIAcuity Digital PCR System and the QIAcuity Nanoplates.
Would you like to learn more about the product and be contacted by one of our dPCR specialists? Sign in here, and we will get in touch with you shortly.
Performance
Superior performance
The QIAcuity Master Mixes for hydrolysis probe-based dPCR use the latest versions of QIAGEN’s high-quality DNA Polymerase. The unique combination of QIAGEN's proprietary and well-proven buffer technology optimized for the Nanoplate microfluidic along with the new QuantiNova DNA Polymerase delivers highly consistent results in terms of sensitivity, reproducibility and efficiency.
Probe-based detection from single to 5-plex with the QIAcuity Probe PCR Kit
The special master mix in the QIAcuity Probe PCR Kit enables accurate quantification of up to 5 targets having widely differing abundance in a well of the QIAcuity Nanoplate. This saves time, money, and reduces the amount of sample material needed. Moreover, the duplex or multiplex PCR data obtained is comparable with that obtained from a singleplex PCR.
Reaction stability of up to 100 hours
The QIAcutiy PCR mixes can be stored at 30°C for up to 100 hours without impairing the performance of subsequent reactions. The outstanding stability, even after extended storage at room temperature without the use of any cooling agent, makes the QIAcuity Probe PCR Kit ideal for high-throughput reaction setup and plate-stack handling.
Principle
The QIAcuity Probe PCR Kit delivers singleplex or multiplex, cDNA or gDNA analysis with the highest specificity because of a novel, antibody-mediated, hot-start mechanism. At low temperatures, the QuantiNova DNA Polymerase is kept in an inactive state by the QuantiNova Antibody and a novel additive, QuantiNova Guard, that stabilizes the complex. This improves the stringency of the hot-start and prevents extension of nonspecifically annealed primers and primer–dimers. Within 2 minutes of raising the temperature to 95°C, the QuantiNova Antibody and QuantiNova Guard are denatured, and the QuantiNova DNA Polymerase is activated, enabling the PCR amplification.
The principle of the dPCR reaction in the nanoplates is described here.
Procedure
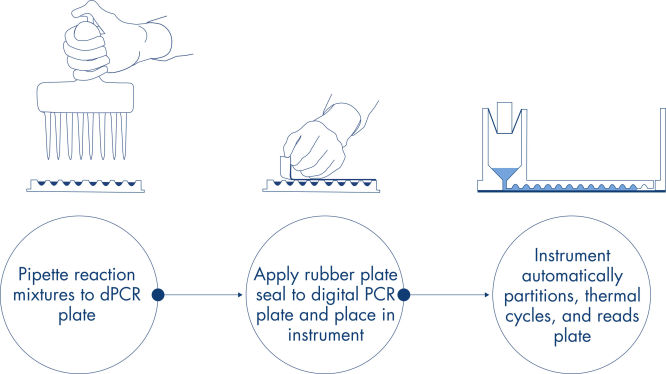
Just like in qPCR experiments, sample preparation includes the transfer of master mix, probes and primers to a 96- or 24-well nanoplate, followed by the addition of samples. The system integrates partitioning, thermocycling and imaging into a single fully automated instrument that takes users from the sample to result in under 2 hours. One can perform analysis on the Suite Software, providing the concentration in copies per microliter of your target sequence as well as for quality control such as positive samples or NTC. This analysis can also be extended to remote computers within the same local area network (LAN).
Applications
The QIAcuity Probe PCR Kit, in combination with the QIAcuity Digital PCR System and the QIAcuity Nanoplates, enable quantitative analysis of cDNA targets and gDNA for use in applications, including:
- Rare mutation detection
- Copy number variation analysis
- Gene expression analysis
- Pathogen detection
- Genotyping
- miRNA research
- Cell and gene therapy
- Residual DNA quantification
- Wastewater monitoring
Supporting data and figures
A simple and rapid plate-based workflow