✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
QIAcuity EG PCR Kit (1 ml)
目录编号 / ID. 250111
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
特点
- 适用于使用 EvaGreen 的染料基数字 PCR 反应
- 3x 浓缩预混液,可加载更大体积样本
- 针对在 QIAcuity 纳米微孔板中的微流体使用优化
- 符合 REACH 法规
产品详情
QIAcuity EG PCR Kit 包含一份针对在 QIAcuity 纳米板中的微流体使用优化的 3x 浓缩即用型预混液。该试剂盒提高了染料法数字 PCR 的特异性和效率,可提供准确的定量分析。EvaGreen 是一种嵌合染料,结合到双链 DNA 上,可提高在QIAcuity dPCR 系统上 gDNA 或 cDNA 定量的准确性。
该试剂盒应与 QIAcuity Digital PCR System和 QIAcuity 纳米微孔板配合使用。
您想要进一步了解该产品并让我们的 dPCR 专家联系您吗?请在这里登记,我们将很快与您联系。
绩效
基于 EvaGreen 染料法的 QIAcuity 预混液中使用了最新版的 QIAGEN 高品质 DNA 聚合酶,展现出卓越的性能。QIAGEN 的专有技术与经过优化验证的专门针对纳米微孔板微流体优化的缓冲液技术的独特结合以及新的 QuantiNova DNA 聚合酶的采用可保证在灵敏度、可重复性及效率等方面高度一致的结果。
使用 EvaGreen 染料法检测
QIAcuity EG PCR Kit 中的独特预混液使您可获得准确的双链 DNA 靶标扩增和定量。该试剂盒中含有 dPCR 分析及纳米板中可分析反应器数目的计数所需的经过优化的参比染料。此外,与相同浓度的 SYBR Green 相比,EvaGreen 可提供更强的荧光信号,在 dPCR 中实现最大化的扩增效率、特异性和灵敏度。
长达 100 个小时的反应稳定性
QIAcuity PCR 混合物可在 30°℃ 下存放长达 100 个小时,而不会影响后续反应的性能。优异的稳定性——即便在室温下不使用冷却剂的情况下存放很长时间后仍是稳定的,使得 QIAcuity EG PCR Kit 成为高通量体系构建及孔板堆叠处理时的理想选择。
原理
QIAcuity EG PCR Kit 采用了新型抗体介导的热启动,因而具有cDNA 或 gDNA 分析具有高的特异性。在低温下,QuantiNova DNA 聚合酶在 QuantiNova 抗体和 QuantiNova Guard(一款具有复合物稳定化作用的新型添加剂)的作用下保持在失活状态。这可提高热启动的严格性并防止非特异性退火引物的延伸及引物二聚体的形成。在温度升高到 95°℃的两分钟内,QuantiNova 抗体和 QuantiNova Guard 将发生变性,QuantiNova DNA 聚合酶将被激活,从而启动 PCR 扩增。
有关纳米微孔板中 dPCR 的反应的原理说明,请参见这里。
程序
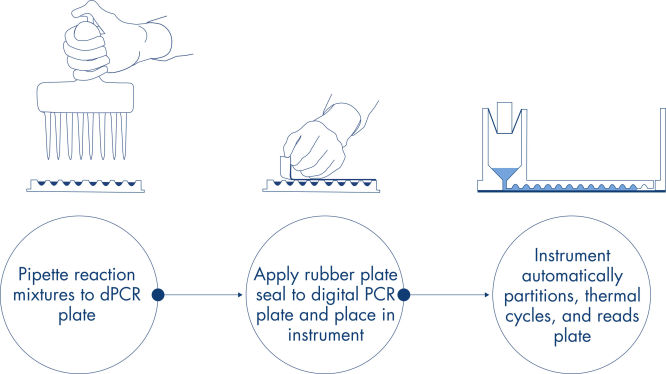
与qPCR 类似,dPCR样本制备包括将预混液、探针和引物转移到一个 96 孔或 24 孔纳米微孔板中,随后添加样本。该系统将微分化、热循环和成像集成到单一全自动仪器上,用户可在 2 小时内实现样本进,数据出。您可在软件套组上执行分析,该软件可针对您的靶序列及质量控制(例如阳性样本或无模板对照)给出以每微升拷贝数表示的浓度。该分析还可在同一局域网上的远程计算机上执行。
应用
QIAcuity EG PCR Kit 与 QIAcuity Digital PCR System 和 QIAcuity 纳米板的结合使用可让您在各种应用中进行 cDNA 靶标和 gDNA 定量分析,这些应用包括:
- 罕见突变检测
- 拷贝数变异分析
- 基因表达分析
- 病原体检测
- 基因分型
- miRNA 研究
辅助数据和图表
简单快速的基于孔板的工作流