PAXgene Blood DNA Kit
全血からのゲノムDNA(最高500 µg)精製
PAXgene Blood DNA Kit
全血からのゲノムDNA(最高500 µg)精製
PAXgene Blood DNAシステムは、採血、安定化、輸送用のパクスジーンDNA採血管(日本ベクトン・ディッキンソン株式会社より入手可能、cat. no. 761115)と、DNA精製用に1本の遠心管で調製できるPAXgene Blood DNA Kitで構成されています。
The fully automated QIAsymphony PAXgene Blood ccfDNA Kit procedure generates ccfDNA yields comparable to the manual QIAamp Circulating Nucleic Acid Kit procedure (see figure High performance, comparable to QIAamp Circulating Nucleic Acid Kit). Since yield is strongly dependent of donor, individual yields may vary. Onboard measures of the QIAsymphony SP minimize cross-contamination.
The workflow begins with a centrifugation step that separates plasma from the cellular fraction of the whole blood collected into PAXgene Blood ccfDNA Tubes. The plasma is then carefully transferred into a second tube. A second centrifugation step can be carried out optionally to remove any traces of buffy coat. The pure plasma is then transferred to a third tube which is loaded into the QIAsymphony SP.
In the QIAsymphony SP, plasma proteins are digested by proteinase K while ccfDNA binds to the surface of magnetic beads (see figure The QIAsymphony SP processes samples containing magnetic particles). Depending on the chosen protocol line, predominantly small ccfDNA fragments (STA protocol line) or small and large ccfDNA fragments (LAF protocol line) are isolated. Three wash steps ensure contaminant removal. Finally, ccfDNA is eluted from the magnetic particles and is ready for use in downstream applications (see figure Two protocol lines of the QIAsymphony PAXgene Blood ccfDNA Kit for different research needs).
DNAは次のような様々なダウンストリームアプリケーションに使用可能です。
PCRおよびリアルタイム定量PCR
サザンブロット法
SNPジェノタイピング
薬理ゲノミクス研究
SNPディスカバリーとバリデーション
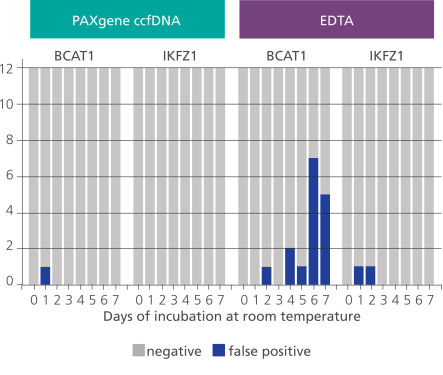
Whole blood of 4 healthy donors was collected into either EDTA or PAXgene Blood ccfDNA Tubes. Blood was left at room temperature for up to 7 days. Each day, ccfDNA was isolated from samples, bisulphite converted (Epitect FAST kit, QIAGEN) and analyzed with the GEMINI assay (Clinical Genomics) based on 2 methylated (BCAT1 and IKZF1) and 1 control (ACTB) target. Three PCR replicates per target sequence were analyzed. ccfDNA from blood stored in EDTA tubes generated more false positives than from blood stored in PAXgene Blood ccfDNA Tubes (data courtesy of Clinical Genomics).
| 特徴 | 仕様 |
|---|---|
| applications | Wide spectrum of research applications, including analysis of cancer tumor DNA and non-invasive prenatal testing |
| format | Magnetic beads |
| inputvolume | 2.4 ml or 4.8 ml plasma |
| throughput | 192 samples per working day and instrument |
| timeperrun | 96 samples of 4.8 ml plasma in 5 h 40 min |
| processing | Manual: plasma centrifugation Automated: QIAsymphony SP |
| shelflife | 6 weeks for an open reagent cartridge |
| elutionvolume | 60 µl for the STA protocol line 60, 100 or 150 µl for the LAF protocol line |